Hermes Medical Solutions receives 510(K) FDA Clearance for new version of Hermia Voxel Dosimetry
Stockholm, Sweden – August 14, 2025 – Hermes Medical Solutions (HMS), global market leader in molecular imaging and dosimetry software solutions, receives 510(k) FDA Clearance for the new version of Hermia Voxel Dosimetry. The product has previously been CE-marked.
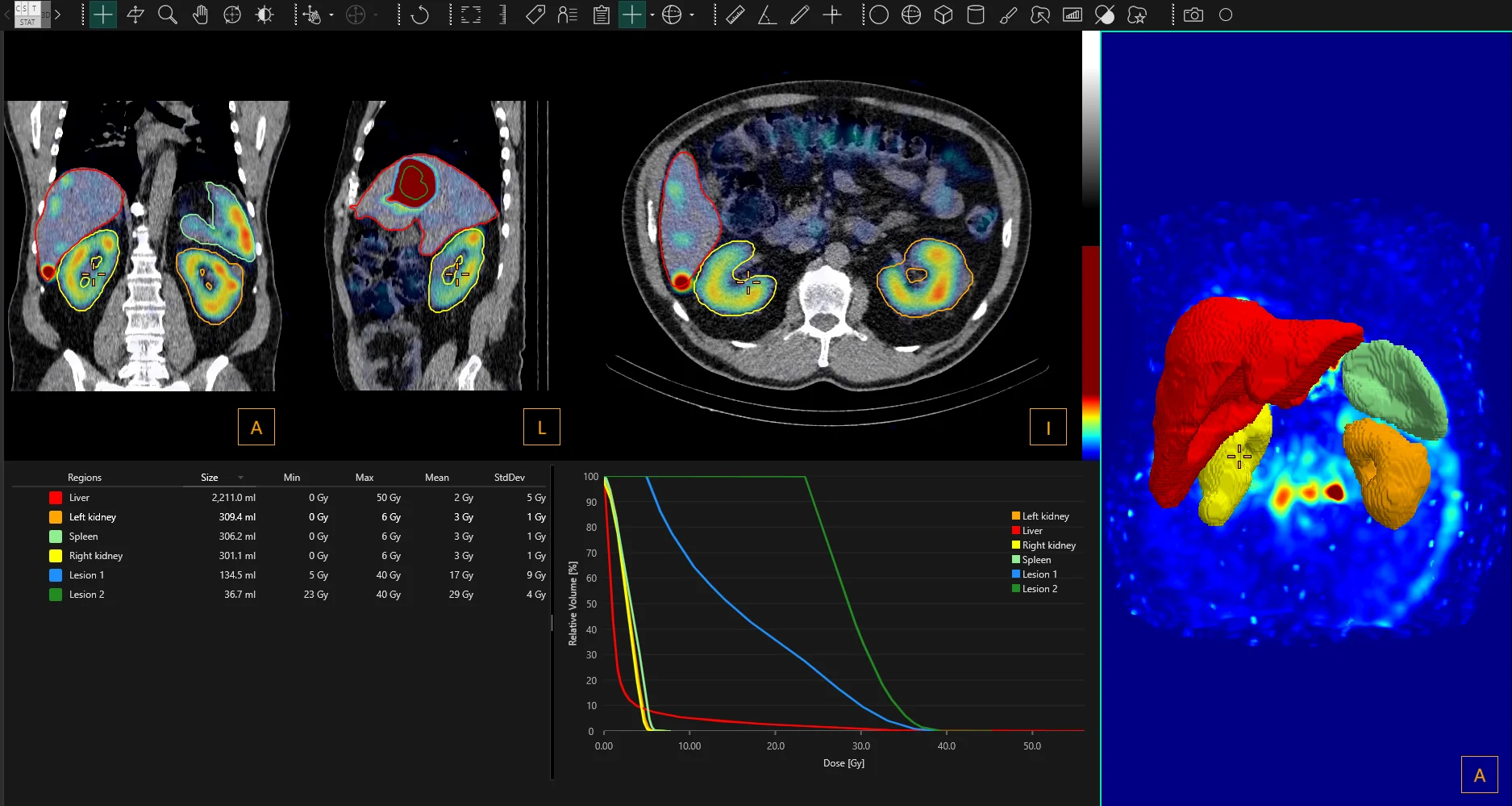
“The new release of the Hermia Voxel Dosimetry is a big step forward in personalized dosimetry for theranostics. It includes additional isotopes, automated workflows, and uses the advanced Hermia Monte Carlo dose calculations. It will enable fast and easy calculations of dose received thanks to the efficient organ segmentation with machine learning algorithm,” said Tom Francke, CEO of Hermes Medical Solutions.
Hermia Voxel Dosimetry introduces several new features* intended to improve its dosimetry aspect and simplify workflows. It is furthermore optimized to calculate dose maps faster than prior versions.
Discover below some of the highlights of the latest version of Hermia Voxel Dosimetry, which has just been FDA-cleared:
Time saving features
- Semi-automatic organ segmentation (with machine learning-based segmentation algorithm)
- Improved ability to fill in information automatically from header
- Reduced calculation times
- Automated workflow configuration options
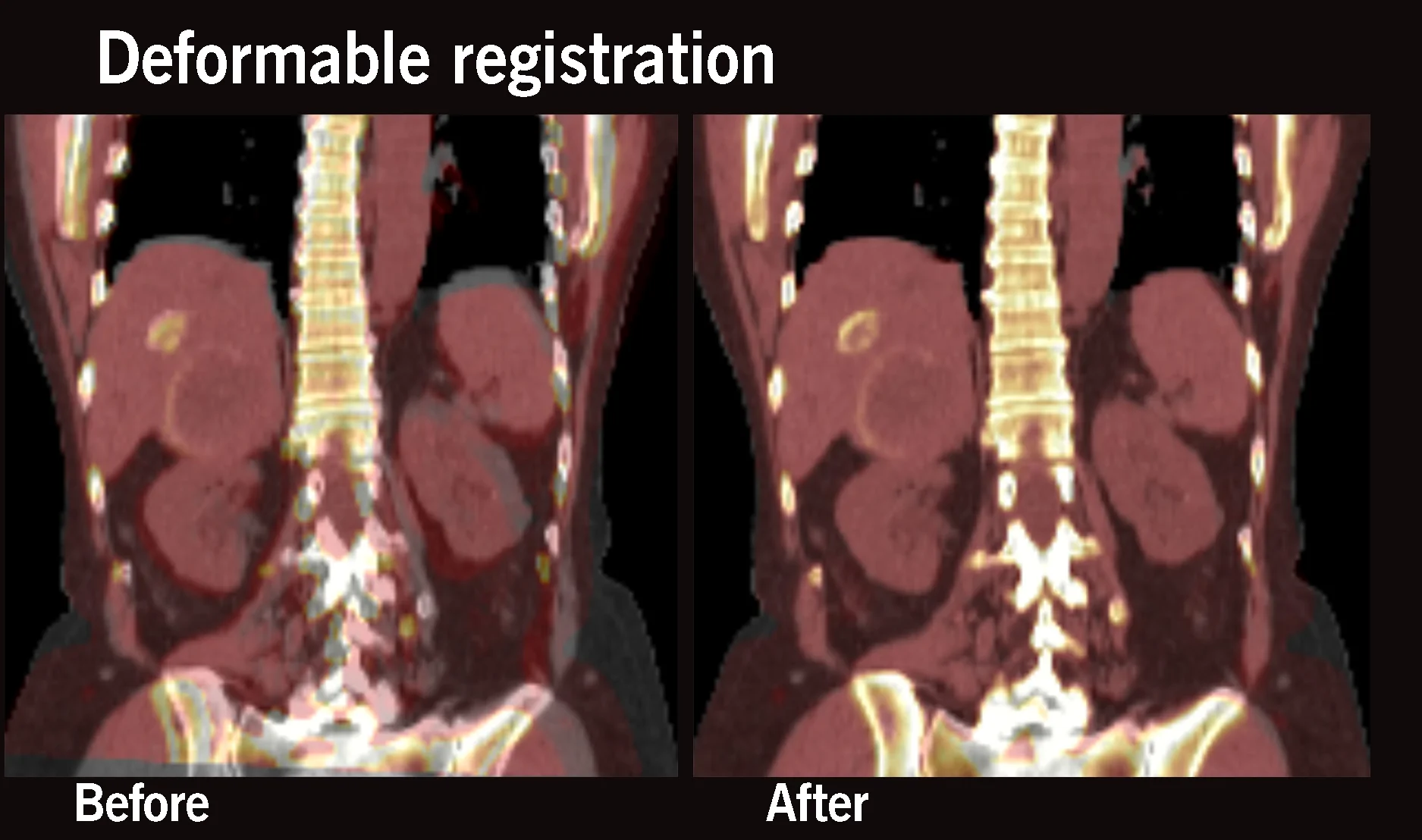
Enhancements
- Deformable registration for enhanced alignment accommodating patient movement and breathing corrections
- Reduced sensitivity to noise
- Improved compatibility with Spectrum Dynamics Quantitative SPECT
- New features for research use
-
- Dose rate calculations
- Add your own isotope
- Surrogate isotope calculations
-
*May be subject to regulatory clearance in your market.
About Hermes Medical Solutions
Since its establishment in 1976, in Stockholm, Sweden, Hermes Medical Solutions continuously innovates to enable faster and more personalized diagnosis and therapies in molecular imaging. Hermes Medical Solutions was first to develop SPECT reconstruction software and dual-head whole-body scanning and first to introduce medical image fusion software for combined viewing of images from different scanners. With Hermia, we empower healthcare professionals with state-of-the-art software for all clinical scenarios into ONE vendor-neutral software suite. Combining leadership in innovation for NM/MI software with customer-driven service is our mission and our success lies in our close and longstanding collaboration with our customers to meet their software, support, and service needs. The result is improved quality in patient management and decision support for thousands of healthcare providers and their patients worldwide.
www.hermesmedical.com
Contact
Bridget Watson
Chief Marketing Officer
Hermes Medical Solutions AB (HQ)
Strandbergsgatan 16
112 51 Stockholm, Sweden
Tel: +46 76 076 47 28
bridget.watson@hermesmedical.com